The world of uncertainty
- Nagaprasad Vr

- Jul 18, 2020
- 1 min read
Uncertainty principle was one of the important principles of quantum physics , without this principle quantum mechanics would have suffered a huge setback and failures. Heisenberg made some assumptions and experiments to discover this principle.
According to Heisenberg’s uncertainty principle we cannot measure both position and momentum of a particle simultaneously . If we try to measure position accurately then accuracy of measuring momentum becomes very low . Therefore while we are measuring two quantities in quantum mechanics we should be careful with the measurements and consider uncertainty principle
Delta p is the uncertainty in momentum and Delta x is the uncertainty in position
Their product is greater than or equal to h bar/2, h bar is reduced plank constant =h/2π
Imagine an electron present inside an atom what would be the maximum uncertainty in finding the position of the electron.
It would be the diameter of the atom , because the electron has to be present somewhere inside the atom right?
If measurements were made considering classical principles , theories of the micro world entities like electrons photons neutrons and other fundamental quantities would lead to so much error that it would be pointless to make such measurements



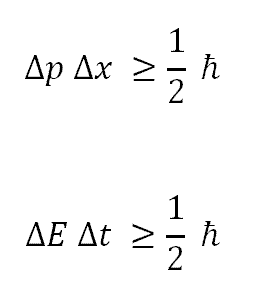


Comments